Pigment Kırmızı 170
| Ürün adı | PIGMENT RED 170 |
| Eş anlamlı | C.I.Pigment Red 170; C.I.PR170; PR170; P.R.170 |
| CI | 12475 |
| CAS NUMARASI. | 2786-76-7 |
| EINECS | 220-509-3 |
| Moleküler ağırlık | 454.48 |
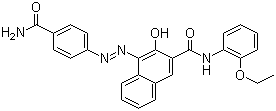
| Moleküler formül | C26H22N4O4 |
| Renk | kırmızı toz |
![]()
Moleküler yapı formülü:
Fastness Properties of Pigment Red 170:
| Işık hızı | 5 |
| Isı Direnci (℃) | 180 |
| Su direnci | 5 |
| Yağ Direnci | 4 |
| Asit Direnci | 5 |
| Alkali Direnci | 5 |
| Alkol Direnci | 4-5 |
Ana uygulama: Su bazlı mürekkep, Ofset mürekkep, Solvent bazlı mürekkep, Plastik, Boya, Tekstil baskı
Farklı müşterilerin ihtiyaçlarını karşılamak için çeşitli pigment sınıfları ve özelliklerimiz var, buna göre tavsiye edebilmemiz için lütfen uygulamanızı ve gereksinimlerinizi belirtin. E-posta: sy@sypigment.com
Product Description of Pigment Red 170:
Pigment Red 170 has stronger than Pigment Red 210, it is a neutral red color and has two crystal forms; the transparent type is blue-red and has a light fastness of level 6, and the non-transparent type has a light fastness of level 7, high hiding power and more stable to solvents; Novoperm Red F2RK The specific surface area of 70 is 23m2/g; it is mainly used in coatings, and can be mixed with molybdenum chrome orange and quinacridone.Mainly used in coatings and solvent printing inks, water-based printing inks, textile printing and dyeing, etc.
Pigment red 170 or PR170 is an organic pigment extensively used in automotive coatings and painting.
It is produced synthetically by converting p-aminobenzamide into the corresponding diazonium compound followed by coupling with 3-hydroxy-2-naphththoic acid (2-ethoxy)anilide ("Naphtol AS-PH" dye precursor).
In the solid state the hydrazo tautomer forms and several crystal structures exist. In the initial α polymorph the molecules are arranged in a herringbone pattern with extensive hydrogen bonding. The φ polymorph is more dense and more stable and produced industrially by thermal treatment in water at 130°C under pressure. In this phase the molecules are planar and arranged in layers. Extensive hydrogen bonding exists within the layer but between layers the only interactions are Van der Waals forces. Dense crystal structures are preferred for pigments used in coatings because in the event of photochemical decomposition the fragments are locked in place and are able to recombine. Research shows that by replacing the ethoxy group in this compound by a methoxy group the crystal structure is less stable and in the final application and the color fades more easily. By careful selection of substituents it is possible to optimize crystal structure and improve optical properties.
TDS (Pigment Red 170) MSDS (Pigment Red 170)Eş anlamlı
- C.I. Pigment Red 170
- 2786-76-7
- PIGMENT RED 170
- UNII-54O6PK8790
- 54O6PK8790
- 4-[(4-carbamoylphenyl)diazenyl]-N-(2-ethoxyphenyl)-3-hydroxynaphthalene-2-carboxamide
- 4-[[4-(Aminocarbonyl)phenyl]azo]-N-(2-ethoxyphenyl)-3-hydroxynaphthalene-2-carboxamide
- CI Pigment Red 170
- 2-Naphthalenecarboxamide, 4-((4-(aminocarbonyl)phenyl)azo)-N-(2-ethoxyphenyl)-3-hydroxy-
- 2-Naphthalenecarboxamide, 4-[[4-(aminocarbonyl)phenyl]azo]-N-(2-ethoxyphenyl)-3-hydroxy-
- 4-4-(Aminocarbonyl)phenylazo-N-(2-ethoxyphenyl)-3-hydroxynaphthalene-2-carboxamide
- Permanent Red F 5RK
- Permanent Red F 3RK70
- C.I. Pigment Red 120
- HSDB 3901
- 4-((4-(Aminocarbonyl)phenyl)azo)-N-(2-ethoxyphenyl)-3-hydroxynaphthalene-2-carboxamide
- EINECS 220-509-3
- EC 220-509-3
- SCHEMBL2139449
- DTXSID7029243
- SCHEMBL12954916
- SCHEMBL14560976
- SCHEMBL16191196
- SCHEMBL21468586
- P.R.266F7RK
- 2-Naphtho-o-phenetidide, 4-((p-carbamoylphenyl)azo)-3-hydroxy-
- ZINC33839056
- ZINC100048551
- 4-((4-(Aminocarbonyl)phenyl)azo)-N-(2-ethoxyphenyl)-3-hydroxy-2-naphthalenecarboxamide
- C.I.12475
- 071N997
- W-109137
- Q15425799
- 2-Naphthalene-carboxamide,3-hydroxy-4-(((4-aminocarbonyl)phenyl)azo)-N-(2-ethoxyphenyl)-
- 3-(2-Ethoxyphenylcarbamoyl)-1-[2-(4-carbamoylphenyl)hydrazono]naphthalene-2(1H)-one
- 4-[(E)-(4-carbamoylphenyl)diazenyl]-N-(2-ethoxyphenyl)-3-hydroxy-2-naphthamide
- 2-Naphthalenecarboxamide, 4-(2-(4-(aminocarbonyl)phenyl)diazenyl)-N-(2-ethoxyphenyl)-3-hydroxy-
IUPAC Name: 4-[(4-carbamoylphenyl)diazenyl]-N-(2-ethoxyphenyl)-3-hydroxynaphthalene-2-carboxamide
InChI: InChI=1S/C26H22N4O4/c1-2-34-22-10-6-5-9-21(22)28-26(33)20-15-17-7-3-4-8-19(17)23(24(20)31)30-29-18-13-11-16(12-14-18)25(27)32/h3-15,31H,2H2,1H3,(H2,27,32)(H,28,33)
InChIKey: BGVYDWVAGZBEMJ-UHFFFAOYSA-N
Canonical SMILES: CCOC1=CC=CC=C1NC(=O)C2=CC3=CC=CC=C3C(=C2O)N=NC4=CC=C(C=C4)C(=O)N
| Property Name | Property Value |
| Molecular Weight | 454.5 |
| XLogP3-AA | 5.2 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 7 |
| Exact Mass | 454.1641052 |
| Monoisotopic Mass | 454.1641052 |
| Topological Polar Surface Area | 126 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 724 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |



