Rouge pigmenté 170
| Nom du produit | PIGMENT RED 170 |
| Synonymes | C.I.Pigment Red 170; C.I.PR170; PR170; P.R.170 |
| CI | 12475 |
| N ° CAS. | 2786-76-7 |
| EINECS | 220-509-3 |
| Masse moléculaire | 454.48 |
| Formule moléculaire | C26H22N4O4 |
| Couleur | Poudre rouge |
![]()
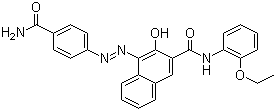
Formule de structure moléculaire :
Fastness Properties of Pigment Red 170:
| Solidité à la lumière | 5 |
| Résistance à la chaleur (℃) | 180 |
| Résistance à l'eau | 5 |
| Résistance à l'huile | 4 |
| Résistance aux acides | 5 |
| Résistance aux alcalis | 5 |
| Résistance à l'alcool | 4-5 |
Application principale : Encre à base d'eau, Encre offset, Encre à base de solvant, Plastique, Peinture, Impression textile
Nous avons différentes qualités et propriétés de pigments pour répondre aux différents besoins des clients, veuillez spécifier votre application et vos exigences afin que nous puissions vous recommander en conséquence. Courriel : sy@sypigment.com
Product Description of Pigment Red 170:
Pigment Red 170 has stronger than Pigment Red 210, it is a neutral red color and has two crystal forms; the transparent type is blue-red and has a light fastness of level 6, and the non-transparent type has a light fastness of level 7, high hiding power and more stable to solvents; Novoperm Red F2RK The specific surface area of 70 is 23m2/g; it is mainly used in coatings, and can be mixed with molybdenum chrome orange and quinacridone.Mainly used in coatings and solvent printing inks, water-based printing inks, textile printing and dyeing, etc.
Pigment red 170 or PR170 is an organic pigment extensively used in automotive coatings and painting.
It is produced synthetically by converting p-aminobenzamide into the corresponding diazonium compound followed by coupling with 3-hydroxy-2-naphththoic acid (2-ethoxy)anilide ("Naphtol AS-PH" dye precursor).
In the solid state the hydrazo tautomer forms and several crystal structures exist. In the initial α polymorph the molecules are arranged in a herringbone pattern with extensive hydrogen bonding. The φ polymorph is more dense and more stable and produced industrially by thermal treatment in water at 130°C under pressure. In this phase the molecules are planar and arranged in layers. Extensive hydrogen bonding exists within the layer but between layers the only interactions are Van der Waals forces. Dense crystal structures are preferred for pigments used in coatings because in the event of photochemical decomposition the fragments are locked in place and are able to recombine. Research shows that by replacing the ethoxy group in this compound by a methoxy group the crystal structure is less stable and in the final application and the color fades more easily. By careful selection of substituents it is possible to optimize crystal structure and improve optical properties.
TDS (Pigment Red 170) MSDS (Pigment Red 170)Synonymes
- C.I. Pigment Red 170
- 2786-76-7
- PIGMENT RED 170
- UNII-54O6PK8790
- 54O6PK8790
- 4-[(4-carbamoylphenyl)diazenyl]-N-(2-ethoxyphenyl)-3-hydroxynaphthalene-2-carboxamide
- 4-[[4-(Aminocarbonyl)phenyl]azo]-N-(2-ethoxyphenyl)-3-hydroxynaphthalene-2-carboxamide
- CI Pigment Red 170
- 2-Naphthalenecarboxamide, 4-((4-(aminocarbonyl)phenyl)azo)-N-(2-ethoxyphenyl)-3-hydroxy-
- 2-Naphthalenecarboxamide, 4-[[4-(aminocarbonyl)phenyl]azo]-N-(2-ethoxyphenyl)-3-hydroxy-
- 4-4-(Aminocarbonyl)phenylazo-N-(2-ethoxyphenyl)-3-hydroxynaphthalene-2-carboxamide
- Permanent Red F 5RK
- Permanent Red F 3RK70
- C.I. Pigment Red 120
- HSDB 3901
- 4-((4-(Aminocarbonyl)phenyl)azo)-N-(2-ethoxyphenyl)-3-hydroxynaphthalene-2-carboxamide
- EINECS 220-509-3
- EC 220-509-3
- SCHEMBL2139449
- DTXSID7029243
- SCHEMBL12954916
- SCHEMBL14560976
- SCHEMBL16191196
- SCHEMBL21468586
- P.R.266F7RK
- 2-Naphtho-o-phenetidide, 4-((p-carbamoylphenyl)azo)-3-hydroxy-
- ZINC33839056
- ZINC100048551
- 4-((4-(Aminocarbonyl)phenyl)azo)-N-(2-ethoxyphenyl)-3-hydroxy-2-naphthalenecarboxamide
- C.I.12475
- 071N997
- W-109137
- Q15425799
- 2-Naphthalene-carboxamide,3-hydroxy-4-(((4-aminocarbonyl)phenyl)azo)-N-(2-ethoxyphenyl)-
- 3-(2-Ethoxyphenylcarbamoyl)-1-[2-(4-carbamoylphenyl)hydrazono]naphthalene-2(1H)-one
- 4-[(E)-(4-carbamoylphenyl)diazenyl]-N-(2-ethoxyphenyl)-3-hydroxy-2-naphthamide
- 2-Naphthalenecarboxamide, 4-(2-(4-(aminocarbonyl)phenyl)diazenyl)-N-(2-ethoxyphenyl)-3-hydroxy-
Nom IUPAC : 4-[(4-carbamoylphenyl)diazenyl]-N-(2-ethoxyphenyl)-3-hydroxynaphthalene-2-carboxamide
InChI : InChI=1S/C26H22N4O4/c1-2-34-22-10-6-5-9-21(22)28-26(33)20-15-17-7-3-4-8-19(17)23(24(20)31)30-29-18-13-11-16(12-14-18)25(27)32/h3-15,31H,2H2,1H3,(H2,27,32)(H,28,33)
InChIKey : BGVYDWVAGZBEMJ-UHFFFAOYSA-N
SOURIRES canoniques : CCOC1=CC=CC=C1NC(=O)C2=CC3=CC=CC=C3C(=C2O)N=NC4=CC=C(C=C4)C(=O)N
| Nom de la propriété | Valeur de la propriété |
| Masse moléculaire | 454.5 |
| XLogP3-AA | 5.2 |
| Nombre de donneurs d'obligations hydrogène | 3 |
| Nombre d'accepteurs de liaison hydrogène | 6 |
| Nombre d'obligations rotatif | 7 |
| Masse exacte | 454.1641052 |
| Masse monoisotopique | 454.1641052 |
| Surface polaire topologique | 126 Ų |
| Nombre d'atomes lourds | 34 |
| Charge formelle | 0 |
| Complexité | 724 |
| Nombre d'atomes isotopiques | 0 |
| Nombre défini de stéréocentres d'atomes | 0 |
| Nombre de stéréocentres d'atomes non définis | 0 |
| Nombre de stéréocentres de liaison définis | 0 |
| Nombre de stéréocentres de liaison indéfinis | 0 |
| Nombre d'unités liées par covalence | 1 |
| Le composé est canonisé | Oui |



